Predict What Would Happen As The Entropy Of A System Approached 100
Predict what would happen as the entropy of a system approached 100. Predict what would happen as the entropy of a system approached 100. All spontaneous change occurs with an increase in entropy of the universe. The entropy change of a system or its surroundings can be negative.
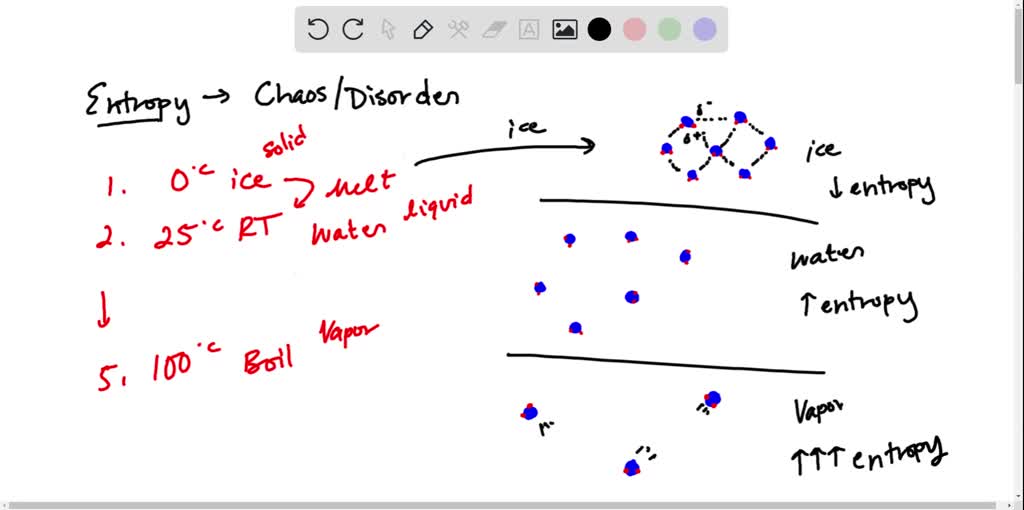
Randomness would become maximized. Identify the principal role of cellular respiration. Technically entropy applies to disorder in energy terms - not just to disordered arrangements in space.
Predict what would happen as the entropy of a system approaches 100. Predict what would happen as the entropy of a system approached 100 A Randomness would become maximized B Heat would decrease C Order. Predict how many molecules of carbon dioxide are produced from the mitochondrial burning of one molecule of glucose.
A All motion would stop. Predict what would happen as the entropy of a system approached 100. In that case we use terminology of change entropy ΔS that is given by.
It loses a phosphate group and is converted to ADP. In particular its the basis for the second law of thermodynamics. Given the definition and characteristics of entropy we can confirm that as the entropy of a system approaches 100 randomness would become maximized.
Randomness would become maximized. ΔS Sfinal state - S initial state Sf - Si. By definition entropy is the scientific idea that corresponds with a state of disorder and randomness.
A system which is more disordered in space will tend to have more disorder in the way the energy is arranged as well. This is known as the increase of entropy principle.
Predict what would happen as the entropy of a system approached 100.
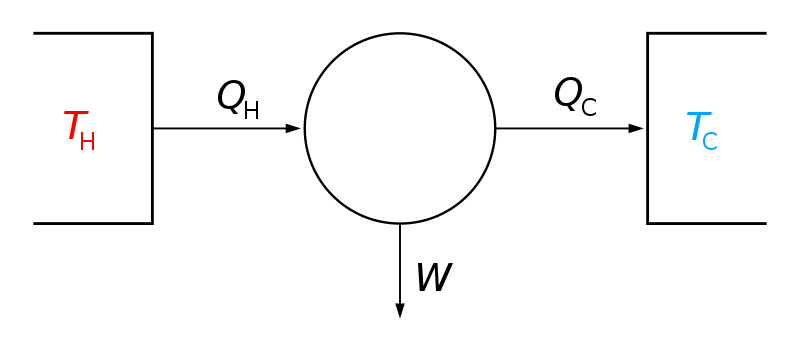
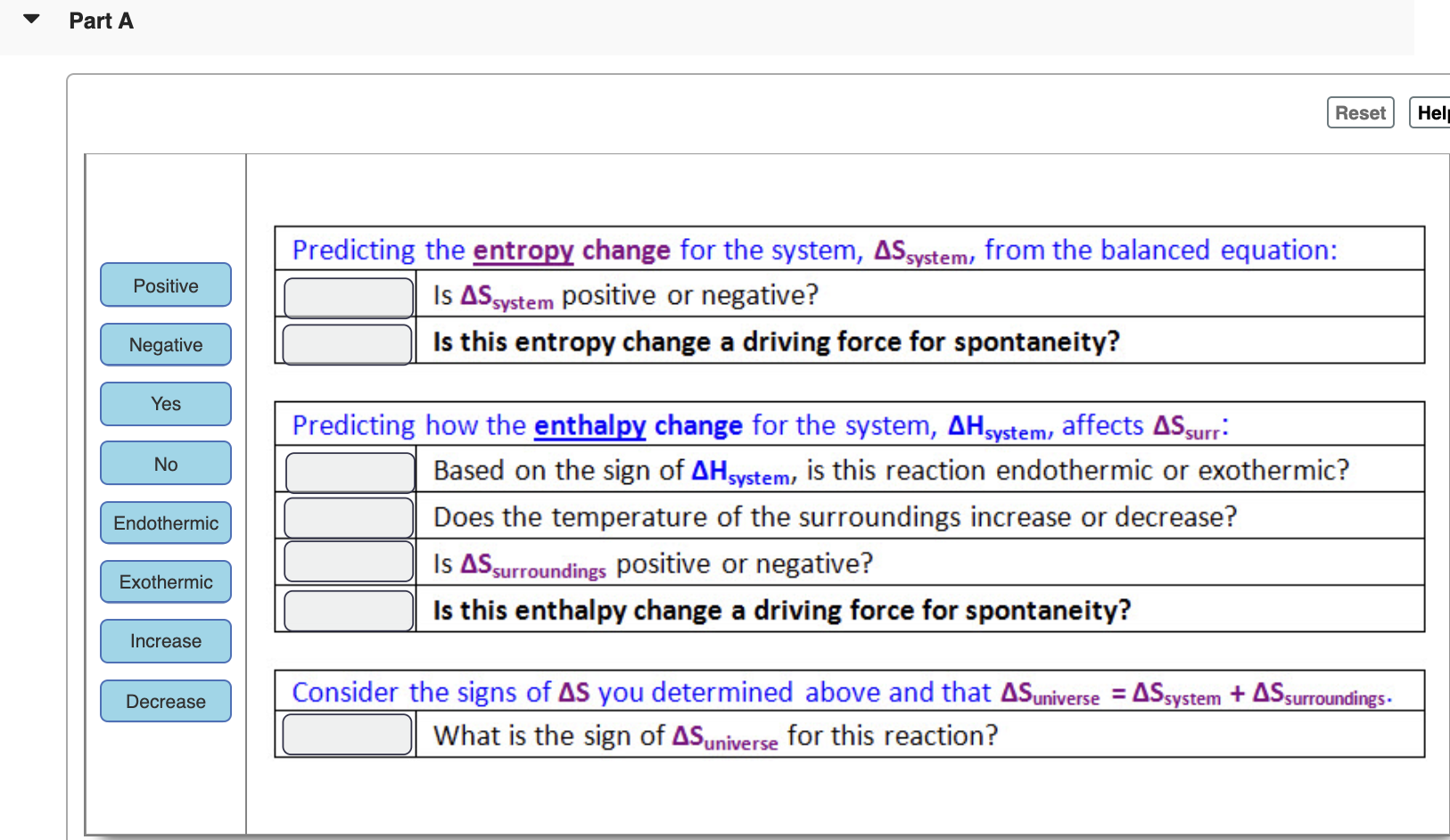
What happens to the ATP molecule after it has been used to do work. Answer 1 of 12. C Order would become maximized. Designating the hotter object as the system and invoking the definition of entropy yields the following. A positive entropy change means an increase in disorder. The entropy change of a system in a process is equal to the amount of heat transferred to it in a reversible manner divided by the temperature at which the transfer takes place. Given the definition and characteristics of entropy we can confirm that as the entropy of a system approaches 100 randomness would become maximized. If ΔS 0 means Sf Si Æ no change in entropy. The arithmetic signs of qrev denote the loss of heat by the system and the gain of heat by the surroundings.
The entropy change of a system in a process is equal to the amount of heat transferred to it in a reversible manner divided by the temperature at which the transfer takes place. Thermodynamics law regarding en. Predict what would happen as the entropy of a system approached 100 A Randomness would become maximized B Heat would decrease C Order. It loses a phosphate group and is converted to ADP. A positive entropy change means an increase in disorder. Given the definition and characteristics of entropy we can confirm that as the entropy of a system approaches 100 randomness would become maximized. Very often we are interested to know the change in entropy of the system going from one state to another in a qualitative manner.





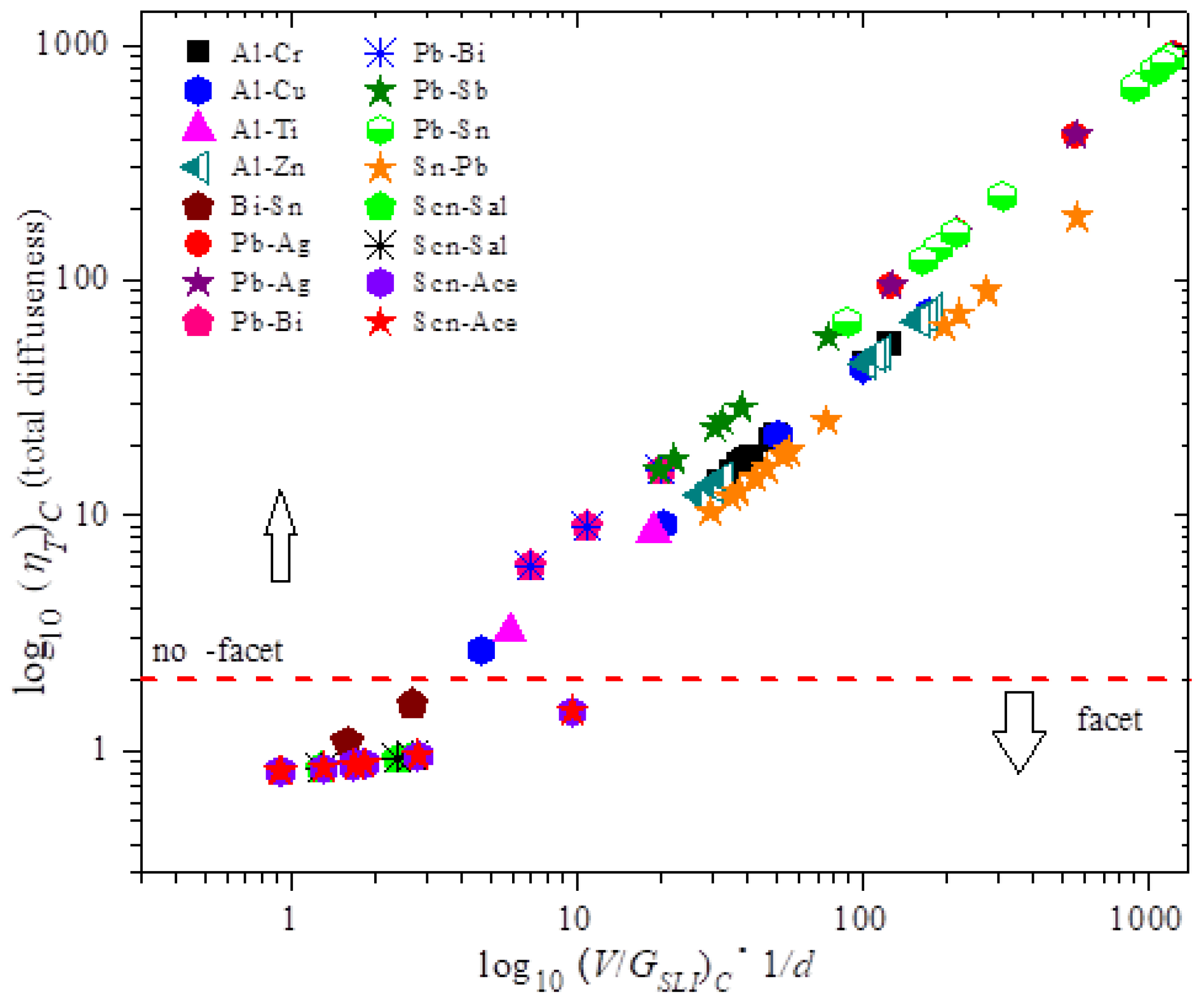
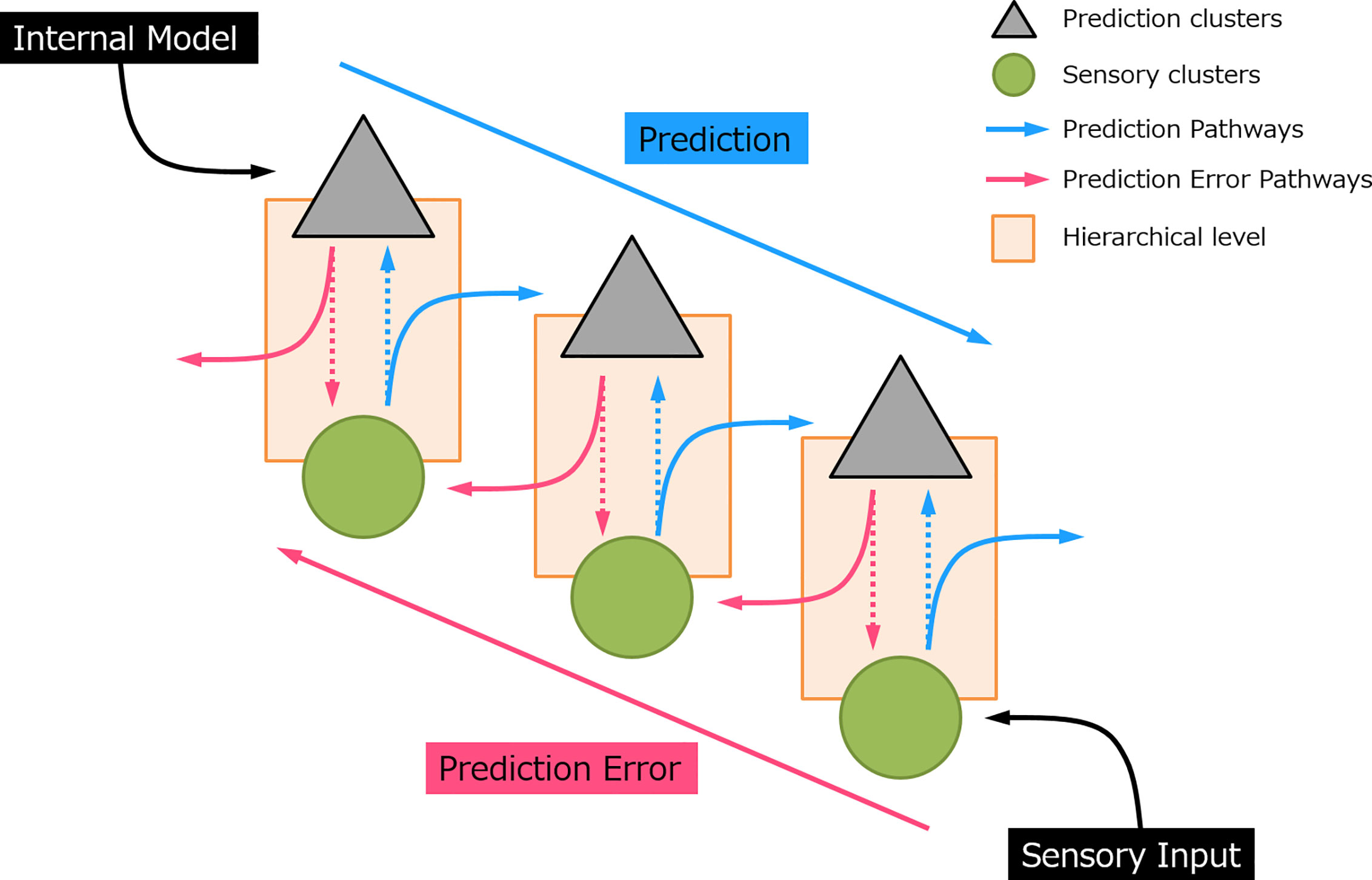
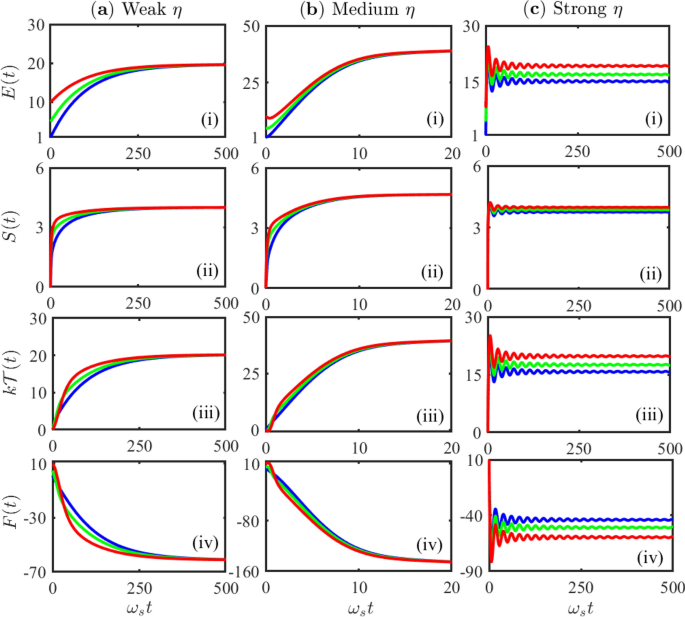












Post a Comment for "Predict What Would Happen As The Entropy Of A System Approached 100"